Archives
Our studies suggest that AdipoR and AdipoR
Our studies suggest that AdipoR1 and AdipoR2 genes are expressed in multiple tissues in the chicken wherein both receptors are likely to mediate the physiological effects of adiponectin. We determined the relative expression of AdipoR1 and AdipoR2 mRNA in various tissues and found that skeletal muscle, adipose tissue and diencephalon were the principal organs where AdipoR1 gene was maximally expressed, while AdipoR2 mRNA expression was the highest in adipose tissue. In contrast to our findings in the chicken, mouse AdipoR1 and AdipoR2 mRNAs have been found to be maximally expressed in the skeletal muscle and liver, respectively (Yamauchi et al., 2003). This may represent species variation in the gene expression of AdipoR1 and AdipoR2. The quantities of AdipoR2 mRNA were found to be significantly higher in liver, skeletal muscle, and ovary than in diencephalon, anterior pituitary gland, kidney, and spleen. AdipoR2 mRNA expressed in the liver is likely to be involved in lipid metabolism, glucose utilization, and gluconeogenesis. AdipoR1 and AdipoR2 expressed in skeletal muscle may be involved in glucose utilization as in mammals. Expression of AdipoR1 and AdipoR2 in the chicken pituitary gland suggests that adiponectin may regulate pituitary hormone secretion. Prolactin and growth hormone have been found to affect AdipoR1 and AdipoR2 gene expression in adipose tissue (Nilsson et al., 2005), and plasma growth hormone concentrations are inversely related to adiponectin concentrations in mice (Berryman et al., 2004) and in humans (Mantzoros et al., 2004).
Expression of adiponectin and its receptors in testis and ovary
We have characterized the expression of adiponectin and its receptors in the testes of broiler breeder chickens (Ocon-Grove et al., 2008). Using RT-PCR and immunohistochemistry, we found that adiponectin, AdipoR1 and AdipoR2 genes are expressed in the chicken testis. We developed chicken-specific GW3965 against adiponectin, AdipoR1 and AdipoR2 and localized adiponectin-, AdipoR1-, and AdipoR2-immunoreactive (ir) cells in the chicken testis (Fig. 3, Fig. 4). Both adiponectin- (Fig. 3) and AdipoR1-ir (Ocon-Grove et al., 2008) cells were localized to the peritubular cells and Leydig cells surrounding the seminiferous tubules in the chicken testis (Fig. 3). Based on distinguishable flattened cell morphology of the peritubular cells, adiponectin and AdipoR1 appear to be expressed in peritubular myoid cells. AdipoR2-immunoreactivity was predominantly observed in the Leydig cells as well as in the adluminal and luminal compartments of the chicken seminiferous tubules (Fig. 4). AdipoR2 immunostaining was observed in the Sertoli cell syncytia suggesting a potential role of adiponectin in regulating Sertoli cell function. In addition, AdipoR2 immunostaining was noticed in the round spermatids, elongating spermatids, and spermatozoa. Based on such widespread AdipoR2 immunostaining throughout the adluminal and luminal compartments of the seminiferous tubule, it is likely that adiponectin affects maturation and differentiation of spermatocytes and therefore potentially influences spermatogenesis. Using quantitative real-time PCR, testicular AdipoR1 and AdipoR2 mRNA quantities were 8.3- and 9-fold higher in adult broiler breeder chickens than prepubertal chickens, respectively, suggesting that sexual maturation is associated with up- regulation of AdipoR1 and AdipoR2 gene expression. A significant elevation of AdipoR1 and AdipoR2 gene expression in sexually mature chickens is likely to be a result of higher metabolic activity related to spermatogenesis, testicular steroid hormone production, and transport of spermatozoa and testicular fluid. In this regard, pla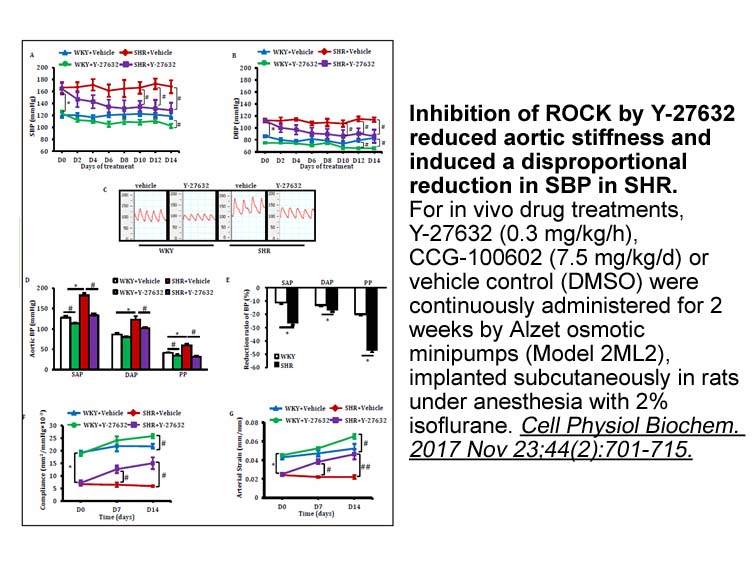 sma adiponectin levels were found to be 4-fold higher in sexually mature versus sexually immature mice (Combs et al., 2003).
Consistent with our findings on expression of adiponectin in the chicken testis, rat testis has been found to express adiponectin in the interstitial Leydig cells (Caminos et al., 2008). Rat testicular adiponectin mRNA levels were modulated by gonadotropins, glucocorticoids, thyroxine, and peroxisome proliferator-activated receptor-γ (Caminos et al., 2008). Furthermore, AdipoR1 were found to be predominantly expressed in the rat seminiferous tubules while recombinant adiponectin treatment significantly inhibited testosterone secretion ex vivo (Caminos et al., 2008). Taken together, adiponectin is likely to influence Leydig cellular metabolism and steroidogenesis, possibly through activation of AMP-activated protein kinase and peroxisome proliferator activated receptors that have been previously identified in mammalian testis tissues (Cheung et al., 2000, Froment et al., 2006). Furthermore, expression of AdipoR2 appears to be critical for testicular function in mice as AdipoR2-deficient knockout mice exhibit reduced testis weight characterized by atrophy of the seminiferous tubules and aspermia, while plasma testosterone levels remained unaffected (Bjursell et al., 2007). Testicular adiponectin may likely act as a paracrine/autocrine factor thereby supplementing blood-borne adiponectin to influence interstitial Leydig cells and seminiferous tubular cells in the chicken. Future studies should focus on elucidating the role of adiponectin signaling on steroidogenesis.
sma adiponectin levels were found to be 4-fold higher in sexually mature versus sexually immature mice (Combs et al., 2003).
Consistent with our findings on expression of adiponectin in the chicken testis, rat testis has been found to express adiponectin in the interstitial Leydig cells (Caminos et al., 2008). Rat testicular adiponectin mRNA levels were modulated by gonadotropins, glucocorticoids, thyroxine, and peroxisome proliferator-activated receptor-γ (Caminos et al., 2008). Furthermore, AdipoR1 were found to be predominantly expressed in the rat seminiferous tubules while recombinant adiponectin treatment significantly inhibited testosterone secretion ex vivo (Caminos et al., 2008). Taken together, adiponectin is likely to influence Leydig cellular metabolism and steroidogenesis, possibly through activation of AMP-activated protein kinase and peroxisome proliferator activated receptors that have been previously identified in mammalian testis tissues (Cheung et al., 2000, Froment et al., 2006). Furthermore, expression of AdipoR2 appears to be critical for testicular function in mice as AdipoR2-deficient knockout mice exhibit reduced testis weight characterized by atrophy of the seminiferous tubules and aspermia, while plasma testosterone levels remained unaffected (Bjursell et al., 2007). Testicular adiponectin may likely act as a paracrine/autocrine factor thereby supplementing blood-borne adiponectin to influence interstitial Leydig cells and seminiferous tubular cells in the chicken. Future studies should focus on elucidating the role of adiponectin signaling on steroidogenesis.